The Redfern Lab
An Overview of Dry Eye
Dry eye is a multifactorial disease of the tears and ocular surface that can result in symptoms of discomfort, visual disturbance, and tear film instability with the potential to damage the ocular surface. It is accompanied by an increase in the osmolarity of the tear film as well as an inflammation of the ocular surface5. Dry eye affects 14%-33% of the adult population worldwide and is the second most common eye problem seen in the clinic10, 12. This syndrome can reduce visual function and quality of life and often arises either from a decrease in tear fluid production and/or a change

in its composition. Dry eye is an incredibly diverse condition, owing its symptoms to a diversity of disorders such as keratoconjunctivitis sicca, dysfunctional tear syndrome, lacrimal keratoconjuctivitis, evaporative tear deficiency, aqueous tear deficiency, and LASIK-induced neurotrophic epitheliopathy as well as a plethora of other syndromes and dysfunctions including faulty lacrimal glands, inflammation of the meibomian glands, inflammation of the ocular surface or conjunctiva, any disease process that alters the components of the tears, an increase in the surface of the eye as a result of a cosmetic surgery that alters the opening of the eyelid, it can be a side effect of medication, skin disease, glandular disease, pregnancy, hormone imbalances, chemical or thermal burns, infrequent blinking as a result of habit or computer/television screen usage, excessive or insufficient vitamin intake, loss of sensation in the cornea due to long-term contact lens wear, nocturnal lagophthalmos (sleeping with your eyes partially open), and immune system disorders such as Sjögren’s, lupus, and rheumatoid arthritis.
With such a diverse array of causal effects, it is no wonder that an estimated 3.2 million women and 1.68 million men age 50 and above are affected by dry eye syndrome in the United States alone9. In addition 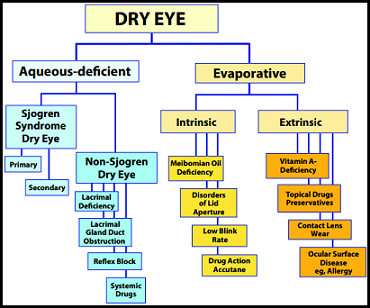 dry eye is quite often exacerbated by age, and each year in the United States more than 2.5 million eye injuries occur that may cause or increase the symptoms of dry eye 15. Exacerbating this issue, dry eye is often misdiagnosed or the disease underlying the issue is ignored leaving the dry eye symptoms to be managed as best they can.
dry eye is quite often exacerbated by age, and each year in the United States more than 2.5 million eye injuries occur that may cause or increase the symptoms of dry eye 15. Exacerbating this issue, dry eye is often misdiagnosed or the disease underlying the issue is ignored leaving the dry eye symptoms to be managed as best they can.
Dry eye is a common multifactorial inflammatory condition that results in ocular discomfort and those who suffer from it are three times more likely to report problems with common daily activities. These sufferers of evaporative dry eye often complain of burning, itching, scratching, or grittiness of the eye that gets worse as the day progresses. Dry eye can make it difficult to perform a number of daily activities, such as reading, driving a car, or using a computer for an extended period of time, and it can reduce ones tolerance for controlled environments such as those of an airplane or an office building7. In extreme cases dry eye can even cause one’s job performance to decrease dramatically or stop one from being able to take part in and enjoy a number of hobbies and past times. All told, the direct annual cost for patients seeking medical care due to dry eye leaves an overall burden of $3.84 billion dollars on the US health care system. Though the indirect costs of dry eye, due primarily to diminished productivity and absenteeism, pays a far larger toll on society, averaging a total of $55.4 billion dollars annually in lost productivity18.
Modality and Expression of the Disease
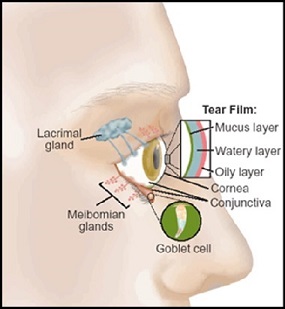 Dry eye occurs due to an irregularity in one or more of the three layers that make up the thin film of tears that coats ones eye throughout the day, and in order to appreciate the issue it is important that one understands the function and make-up of these three layers. The most superficial layer of the tear film is comprised of a very thin layer of lipids, these lipids are produced by the meibomian glands lining the inside of the eyelids and are responsible for mitigating the rate and extent of evaporation of the watery layer underneath. This watery layer, known as the aqueous layer of the tear film, is by and large the thickest of the three layers of the tear film. The lacrimal gland, located under the upper lid, produces this watery layer however this is where the proteins, cytokines, enzymes and other biomolecules of the ocular surface tend to interact. While this layer of the tear film is responsible for keeping the eye moist, comfortable, and free of debris and foreign objects, it is important that the environment within this aqueous layer remain relatively stable in order to maintain its intended function. The last and innermost layer of the tear film consists of a layer of mucins and is the thinnest portion of the tear film, though it is by no means the least important. Mucins released by the goblet cells in the conjunctiva are dragged across the ocular surface each blink and allow the overlying aqueous layer to evenly spread over the surface of the eye. The area of the body responsible for maintaining the tear film is an interdependent set of lacrimal system components known as the Lactrimal Functional Unit (LFU)13. The LFU is comprised specifically of the lacrimal glands, ocular surface (Cornea and conjunctiva), meibomian glands, lids, and the sensory and motor nerves that connect them. Any issue compromising one of these components has the potential to cause a disturbance in the tear film stability through a decrease in tear secretion, delayed tear clearance, and an altered tear composition5. The pathogenesis behind a compromised LFU can be tricky to pinpoint, evidence suggests that dry eye is a multifactorial issue. Factors such as age, genetic predisposition, gender and hormonal levels, exposure to environmental stress, nutrition, the status of one's immune system and exposure to pathogens, and even the status of the ocular nerves can alter the cellular and molecular structure and function of individual components in this system 5. Knowing all of this however, what have we been able to ascertain thus far about the underlying mechanisms of dry eye and the ocular surface environment?
Dry eye occurs due to an irregularity in one or more of the three layers that make up the thin film of tears that coats ones eye throughout the day, and in order to appreciate the issue it is important that one understands the function and make-up of these three layers. The most superficial layer of the tear film is comprised of a very thin layer of lipids, these lipids are produced by the meibomian glands lining the inside of the eyelids and are responsible for mitigating the rate and extent of evaporation of the watery layer underneath. This watery layer, known as the aqueous layer of the tear film, is by and large the thickest of the three layers of the tear film. The lacrimal gland, located under the upper lid, produces this watery layer however this is where the proteins, cytokines, enzymes and other biomolecules of the ocular surface tend to interact. While this layer of the tear film is responsible for keeping the eye moist, comfortable, and free of debris and foreign objects, it is important that the environment within this aqueous layer remain relatively stable in order to maintain its intended function. The last and innermost layer of the tear film consists of a layer of mucins and is the thinnest portion of the tear film, though it is by no means the least important. Mucins released by the goblet cells in the conjunctiva are dragged across the ocular surface each blink and allow the overlying aqueous layer to evenly spread over the surface of the eye. The area of the body responsible for maintaining the tear film is an interdependent set of lacrimal system components known as the Lactrimal Functional Unit (LFU)13. The LFU is comprised specifically of the lacrimal glands, ocular surface (Cornea and conjunctiva), meibomian glands, lids, and the sensory and motor nerves that connect them. Any issue compromising one of these components has the potential to cause a disturbance in the tear film stability through a decrease in tear secretion, delayed tear clearance, and an altered tear composition5. The pathogenesis behind a compromised LFU can be tricky to pinpoint, evidence suggests that dry eye is a multifactorial issue. Factors such as age, genetic predisposition, gender and hormonal levels, exposure to environmental stress, nutrition, the status of one's immune system and exposure to pathogens, and even the status of the ocular nerves can alter the cellular and molecular structure and function of individual components in this system 5. Knowing all of this however, what have we been able to ascertain thus far about the underlying mechanisms of dry eye and the ocular surface environment?
In order to discuss the specifics of dry eye we will be narrowing our focus by concentrating specifically on evaporative dry eye which accounts for 35-45%6, 14, 16, 17 of all dry eye cases. Though it is important to note that tear hyperosmolarity and tear film instability, the two major facets of evaporative dry eye, can be seen as core mechanisms at the center of the dry eye process and can be initiated, amplify, and potentially change the characteristics of any form of dry eye over time 2. Tear film hyperosmolarity, and consequent ocular surface inflammation, 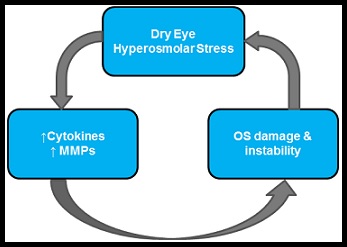 is thought to be an initiating factor for a dry eye related immune response by activating ocular surface epithelial and stromal cells to elicit a cascade of inflammatory events, through the MAP kinases and NFkB signaling pathways, that creates an increase in proinflammatory cytokines and matrix metalloproteinases (MMPs) 4. While the specific mechanisms behind this activation are unknown, the release of inflammatory cytokines and MMPs often starts a vicious cycle of dye eye that is difficult to combat1. This cycle starts with the hyperosmolarity of the ocular surface which causing hyperosmolar stress and the up-regulation of inflammatory cytokines and MMPs. Hyperosmolarity occurs as the aqueous layer of the tear film either evaporates too rapidly, is not replenished quick enough, or contains an unregulated amount of bio-molecules and/or electrolytes. These unregulated bio-molecules then lead to ocular surface damage that can cause further hyperosmolar stress, reinforcing and perpetuating the issue. In the initial stages of dry eye, the introduction of ocular surface damage or inflammatory stress results in reflex tear stimulation in the lacrimal gland which can initially be enough to compensate for tear film hyperosmolarity 11 as tear fluid secreted from the lacrimal gland is slightly hypotonic in nature 3. While this can initially offset the osmolarity of the tear film, which can become hyperosmotic due to evaporation and tear film break up, as chronic dry eye disease sets in corneal sensitivity is impaired and reflex tearing is diminished resulting in an even greater tear film instability exacerbating the disease.
is thought to be an initiating factor for a dry eye related immune response by activating ocular surface epithelial and stromal cells to elicit a cascade of inflammatory events, through the MAP kinases and NFkB signaling pathways, that creates an increase in proinflammatory cytokines and matrix metalloproteinases (MMPs) 4. While the specific mechanisms behind this activation are unknown, the release of inflammatory cytokines and MMPs often starts a vicious cycle of dye eye that is difficult to combat1. This cycle starts with the hyperosmolarity of the ocular surface which causing hyperosmolar stress and the up-regulation of inflammatory cytokines and MMPs. Hyperosmolarity occurs as the aqueous layer of the tear film either evaporates too rapidly, is not replenished quick enough, or contains an unregulated amount of bio-molecules and/or electrolytes. These unregulated bio-molecules then lead to ocular surface damage that can cause further hyperosmolar stress, reinforcing and perpetuating the issue. In the initial stages of dry eye, the introduction of ocular surface damage or inflammatory stress results in reflex tear stimulation in the lacrimal gland which can initially be enough to compensate for tear film hyperosmolarity 11 as tear fluid secreted from the lacrimal gland is slightly hypotonic in nature 3. While this can initially offset the osmolarity of the tear film, which can become hyperosmotic due to evaporation and tear film break up, as chronic dry eye disease sets in corneal sensitivity is impaired and reflex tearing is diminished resulting in an even greater tear film instability exacerbating the disease.
A Deeper Look
Dry eye is a difficult disorder to study, due to the dynamic nature of the ocular surface environment it is important that one take into account all of the variables that may affect protein expression. The eye is the only mucus membrane on the body that is in constant contact with the outside environment, due to this it is constantly needing to renew both the tear film as well as the epithelial cells on the outside of the eye. It is this high turnover rate that makes it so difficult to determine any trends or changes concerning protein expression and immunoresponse. Any changes within the tear film either alter with the diurnal changes in the body or will be washed away and renewed shortly after expression. To make this even more complex, any proteins expressed in the cells are regularly released and broken down in response to stressors or healing patterns.
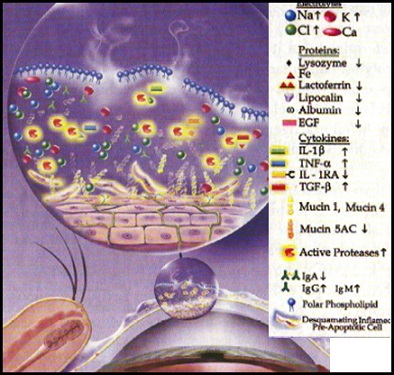 Despite the inherent difficulty in studying the ocular surface, many bio-molecules of interest have been discovered that can be linked to dry eye and inflammation. Aqueous deficient dry eye results in a reduction in tear volume, either due to increased evaporation or reduced tear production, resulting in a hyperosmolar environment on the surface of the eye which stimulates the release of proinflammatory cytokines and MMPs which leads to the recruitment of immune cells as well as a disruption of the ocular surface. Research suggests that the cytokines interleukin-6, 8 and IB (IL6, IL8, and IL-1B) play a major role in the pathology of dry eye. IL-6 acts as a proinflammatory cytokine secreted by T-cells and has been found to play a role in the inflammatory response to disease, infection and damage. Once inflammation has begun, activated macrophages secrete IL-1B which acts as a proprotein playing a part in cell proliferation, differentiation, and apoptosis. IL-8, which is produced by both macrophages and epithelial cells, induces chemotaxis in neutrophils causing them to migrate towards the ocular surface and induces phagocytosis once they arrive. IL-8 can be secreted by any cells with TLRs involved in the innate immune response and is usually released by macrophages, as they are the first to usually recognize antigens.
Despite the inherent difficulty in studying the ocular surface, many bio-molecules of interest have been discovered that can be linked to dry eye and inflammation. Aqueous deficient dry eye results in a reduction in tear volume, either due to increased evaporation or reduced tear production, resulting in a hyperosmolar environment on the surface of the eye which stimulates the release of proinflammatory cytokines and MMPs which leads to the recruitment of immune cells as well as a disruption of the ocular surface. Research suggests that the cytokines interleukin-6, 8 and IB (IL6, IL8, and IL-1B) play a major role in the pathology of dry eye. IL-6 acts as a proinflammatory cytokine secreted by T-cells and has been found to play a role in the inflammatory response to disease, infection and damage. Once inflammation has begun, activated macrophages secrete IL-1B which acts as a proprotein playing a part in cell proliferation, differentiation, and apoptosis. IL-8, which is produced by both macrophages and epithelial cells, induces chemotaxis in neutrophils causing them to migrate towards the ocular surface and induces phagocytosis once they arrive. IL-8 can be secreted by any cells with TLRs involved in the innate immune response and is usually released by macrophages, as they are the first to usually recognize antigens.
In addition to these cytokines, MMPs such as MMP3 and 9 are released in response to inflammation. These enzymes target gelatin and collagen substrates and are responsible for cleaning up debris caused by dry eye inflammation and damage. However, it is believed that when MMPs are present for too long or in too high of a concentration, they can start a pattern of non-specific targeting that can break down and damage the extracellular matrix of the epithelium. This damage to the extracellular matrix then leads to the recruitment of macrophages that attempt to clean up the debris caused by the MMPs, thus adding to the inflammatory pattern of dry eye.
Knowing this however, still leaves us short of curing the underlying factors behind dry eye. While one may think that the solution to this issue may be as simple as suppressing and expressing target bio-molecules on the ocular surface, these same molecules also play a significant role in the suppression of bacterial, viral, and fungal infections and under normal conditions aid in the routine maintenance of the eye. In attempting to alleviate the underlying pathology of dry eye disease we must be careful to maintain the body's ability to fight off foreign contamination and the build-up of debris and electrolytes on our ocular surface.
The science of dry eye is still fairly young, and there is still much to learn about the underlying pathology of the disease. And while we do know some of what factors into an unhealthy ocular surface environment, there are still many aspects yet unexplored. It is our goal in researching dry eye to find these remaining elements, to tease away the mystery behind the only mucous membrane on our bodies that is in constant contact with the external environment, and to uncover the immune responses therein.
- Baudoin C. The vicious circle in dry eye syndrome: a mechanistic approach. J Fr Ophtalmol 2007;30:239-46
- belson MB, Smith L, Chapin M. Ocular allergic disease: Mechanisms, disease subtypes, treatment. Ocul Surf 2003;1:127-49
- Bron AJ, Tiffany JM, Yokoi N, Gouveia SM. Using osmolarity to diagnose dry eye: a compartmental hypothesis and review of our assumptions. Adv Exp Med Biol 2002;506(PtB):1087-95
- De Paiva CS, Corrales RM, Villarreal AL , et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAP K activation in the corneal epithelium in experimental dry eye. Exp Eye Res 2006;83:526-35
- DEWS The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75–92.
- Mathers WD,Lane JA,Sutphin JE,et al.Model for ocular tear film function.Cornea.1996;15:110-119.
- Miljanovic B, Dana R, Sullivan DA, et al. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409-415.
- Prevalence of Dry Eye Disease Among US Men: Estimates from the Physicians' Health Studies. Arch Ophthalmol. 2009;127:763-8.
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of Dry Eye Syndrome Among US Women. Am J Ophthalmol. 2003;136:318-26.and Schaumberg DA, Dana R, Buring JE, Sullivan DA.
- Schaumberg D.A., D.A. Sullivan, M.R. Dana Epidemiology of dry eye syndrome Adv. Exp. Med. Biol., 506 (2002), pp. 989–998
- Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol 1995;113:1266-70
- Smith J. A., J. Albenz, C. Begley et al., “The epidemiology of dry eye disease: report of the epidemiology subcommittee of the International Dry Eye Workshop,” Ocular Surface, vol. 5, no. 2, pp. 93–107, 2007
- Stern ME, Beuerman RW, Fox RI , et al. The pathology of dry eye; the interaction between the ocular surface and lacrimal glands. Cornea 1998;17:584-9
- Tong L,Chaurasia SS,Mehta JS,et al.Screening for meibomian gland disease:its relation to dry eye subtypes and symptoms in a tertiary referral clinic in Singapore.Invest Ophthalmol Vis Sci.2010;51:3449-3454.
- United States Eye Injury Registry Summary Report, 1998-2002
- Viso E,Gude F,Rodriguez-Ares MT.The association of meibomian gland dysfunction and other common ocular diseases with dry eye:A population-based study in Spain.Cornea.2011;30(1):1-6.
- Yamaguchi M,Kutsuna M,Uno T,et al.Marx line:fluorescein staining line on the inner lid as indicator of meibomian gland function.Am J Ophthalmol.2006;141:669-675.
- Yu Junhua , Asche Carl V, Fairchild Carol. The Economic Burden of Dry Eye Disease in the United States: A Decision Tree Analysis. Cornea. April 2011; 30:0277-3740.